TMS in Australia - New Guidelines for TMS Practice
July 12, 2024 - neurocare clinics Australia
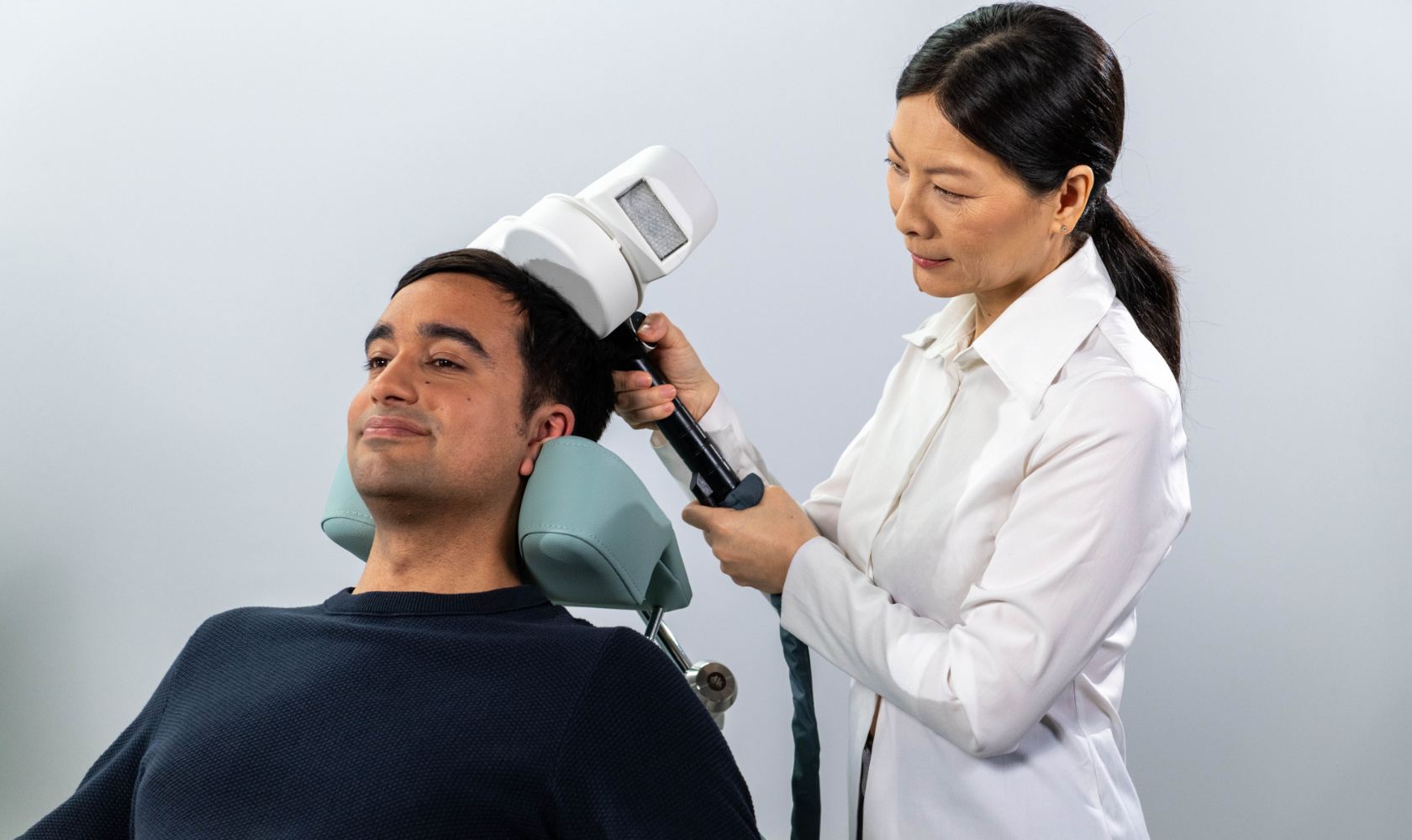
rTMS, also known as TMS, is a type of therapy which provides localised brain stimulation using an electronic magnet. When applied, TMS induces small electrical currents in outer regions of the brain, which causes neurons to fire in the area. It is a popular treatment for depression worldwide because it is non-invasive and has very mild side effects, particularly compared to other depression treatments. This is one of the reasons why TMS is listed on the Medicare Benefits Schedule in Australia for treatment-resistant depression.
The expanding research on TMS for various conditions makes this paper particularly important. The authors, members of the Royal Australian and New Zealand College of Psychiatrists (RANZCP), set the requirements for TMS treatment practice in Australia and New Zealand. They have summarized their recommendations for practice based on global research and practice requirements.
TMS for Depression
Treatment-resistant major depression is the most common condition treated with TMS. Here, “treatment-resistant” means that treatment with medication is not appropriate or has not adequately helped the patient. In these cases, TMS treatment works for between 58-83% of patients.
Other types of depression also give promising results, but the authors were unable to determine efficacy rates for anxious or bipolar depression. In depression with psychosis, it remains important for clinicians to determine whether electroconvulsive therapy (ECT) is more appropriate on a case-by-case basis.
TMS for Schizophrenia
Several studies provide evidence that schizophrenia can be effectively treated with TMS, but not all studies agree, and the effect is not as significant as in depression. Some symptoms of schizophrenia respond better to TMS than others. Due to insufficient evidence and the cost involved, TMS is not currently recommended over conventional treatments for schizophrenia.
TMS for OCD
OCD has been treated by TMS for some time with mixed outcomes. Recent experiments with a “deep TMS” coil, which stimulates in a more widespread fashion across the brain than a conventional coil, are promising. These devices have been approved for use in Australia by the TGA, but RANZCP needs more evidence before recommending them for routine clinical practice.
TMS for Post-Traumatic Stress Disorder
With strong results in the initial studies, TMS treatment for PTSD looks promising. Multiple protocols have been used successfully and now the field is currently trying to reach consensus on one protocol. Unfortunately, RANZCP cannot recommend a protocol yet.
TMS for Anxiety
Meta-analytic research found good evidence that TMS can reduce anxiety reliably. More research is required before consensus.
TMS for Eating disorders
Preliminary research in this field suggests that TMS can address the affective side of eating disorders but perhaps not alleviate behaviour. More extensive research is required to make conclusions for clinical practice here.
TMS for Addiction
Different types of addiction seem to respond differently to TMS treatment. Among behavioural, depressant, and stimulant addiction types, there is evidence that TMS could be effective, but with most research completed only recently, this field is young.
Addiction with comorbid schizophrenia and nicotine addiction in smoking have returned particularly promising results in several TMS studies so far.
TMS for Chronic pain
Many attempts have been made to use TMS to treat chronic pain in the past, and approaches vary heavily. However, the research so far covers many different types of chronic pain and protocols. These protocols do not always work to alleviate chronic pain. However, the RANZCP considers neuropathic pain the most promising target, and TMS over the left-side motor cortex (or left M1) the most promising protocol.
TMS for Post-stroke depression and cognition
When using rTMS to treat people with depression and cognitive problems after having a stroke, recent research found improvements in depression, but not cognitive ability. Some more research is needed before this protocol is elevated to a level suitable for clinical applications.
TMS in other indications
Studies on autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) are limited, and more evidence is needed to draw conclusions. Magnetic e-Resonance Therapy (MERT) is not yet sufficiently supported by research outcomes to be recommended by the RANZCP for clinical practice.
Safety and Risks of TMS
TMS is recognised as a safe therapy with minimal side-effects. It is important to speak to a psychiatrist about risks. RANZCP suggest that the following be considered:
TMS in pregnant women: evidence and theory agree that the risk to a mother and foetus is minimal if not negligible, but programs involving a pregnant person should be monitored closely just in case.
TMS in children and adolescents: currently, TMS is only recommended for people aged 18 and above. It can however be made available to people under 18 after careful assessment, particularly when other treatment options are limited.
TMS in older people: no evidence exists so far to suggest that TMS is less effective or less safe in older people.
TMS in pre-existing conditions: The risk of seizure under TMS is extremely low, but should be considered. Metal or electronic implants which are close to the coil should be assessed for potential interactions.
TMS in people with hearing protection: The TMS machine makes a sharp clicking noise; hearing protection may be advisable for those experiencing discomfort.
Side effects of TMS
TMS side effects are typically very mild. Only 2.5% of people stopped TMS treatment during studies due to side effects, which is lower than people receiving sham TMS (2.7%).
Side effect severity usually reduces over the course of the program. Some people experience:
- Mild discomfort or headache at the stimulation site
- Mild fatigue
- Muscle twitching during stimulation
Rarely, people have reported:
- Seizure. Theoretically possible but almost never observed with proper screening. The highest incidence reported is 0.0075%, with no evidence suggesting increased future seizure risk.
- Manic or hypomanic episode in bipolar patients. Also rare in practice, particularly in individuals taking mood-stabilizing medication.





